Research
Lipoprotein Biosynthesis in Firmicutes
Lipoproteins are abundant, globular proteins located on the surface of bacterial cell membranes. Lipoproteins are anchored to the membrane through an acylated N-terminal cysteine residue, where they serve numerous cellular roles including as cell envelope structural components, in adhesion, in cell-cell interactions, and in the capture and transport of nutrients. The ubiquitous distribution of lipoproteins among bacteria, as well as their unique structure, makes lipoproteins important ligands for bacterial detection by innate immunity. Lipoproteins are recognized by binding to Toll-like receptor 2 (TLR2) on macrophages and other host cells, triggering a pro-inflammatory signaling cascade intended to clear the bacterial infection. While the structure of lipoproteins is otherwise highly conserved across both gram positive and negative bacteria, the acylation pattern can vary even at the species levels within a given genera among Firmicutes. We aimed to uncover the enzymes responsible for specific lipoprotein acylation patterns in Firmicutes. To this End, We have identified three orthogonal N-terminal lipoprotein modification systems: Lit, LnsA/B and LhaT/YvoF/PcrB. We’re currently investigating the overlapping as well as the chemotype-specific roles that might contribute to both general microbial fitness/copper resistance and to immunoevasion.
LPS modifications: AMR and host immunity
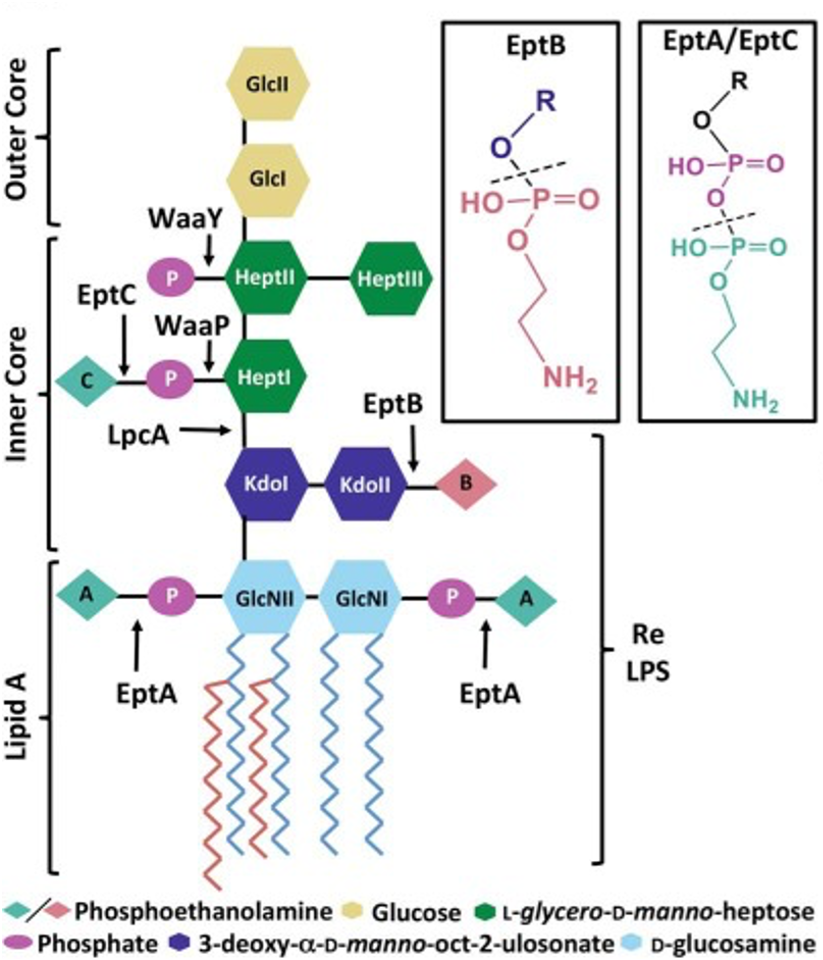
Lipopolysaccharide (LPS) decorates the outer surface of the outer membrane of Gram-negative bacteria. The structure of LPS comprises of 1) the O-antigen, made of repeating units of oligosaccharides, 2) core sugars containing a distinct set of oligosaccharides, and 3) the lipid A moiety through which the molecule anchors into the outer membrane. While the O-antigen and the core sugars are highly variable in composition, the lipid A is the most conserved region of the LPS molecule. The lipid A moiety is characterized by a di-glucosamine backbone with acyl chains appended onto it, as well as the characteristic 1 and 4’ phosphate groups that give the molecule an overall negative charge. Despite being the most conserved region, the lipid A moiety undergoes modifications based on environmental cues that allow the bacteria to survive in stressful environmental conditions, as well as provide resistance against cationic antimicrobials by reducing the overall negative charge of LPS. In our lab, we study the various chemotypes of LPS and their role in eliciting host immune responses as well as their ability to evade antimicrobial peptides. We are also interested in understanding the role of these LPS chemotypes in driving host-microbe interactions in the host gut and its implications in inflammatory disorders such as IBD, Crohn’s disease etc.
Functional genomics in S. aureus
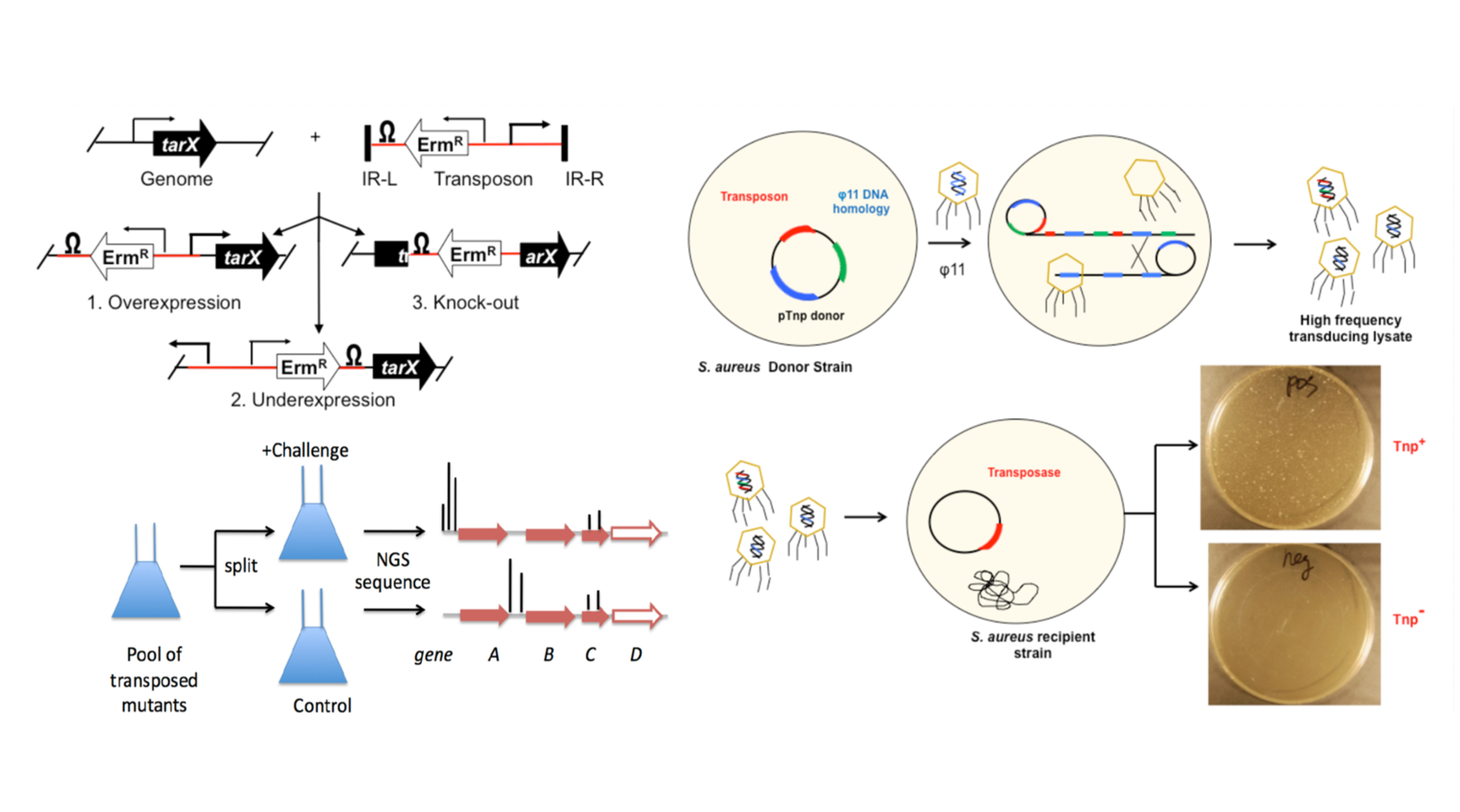
Methicillin resistant Staphylococcus aureus (MRSA) is a leading cause of hospital acquired infections, and ever increasingly outside of the clinic with the rise of hyper virulent community acquired S. aureus lineages. With nearly 30% of the ~3 Mb genome subject to genetic exchange (via plasmids, transposons, bacteriophage, etc.), S. aureus demonstrates remarkable genomic plasticity that confers a high level of adaptability. Certain members within clonal complexes account for a disproportionate number of infections. We are interested in understanding the molecular determinants that impart increased fitness, focusing on contemporary clinical isolates. We have developed a comprehensive phage based delivery transposon system in S. aureus to generate high coverage libraries in diverse strains belonging to widely circulating clonal complexes. Each cassette contains a distinct promoter or transcriptional terminator element of varying intrinsic strength, so a gradient of gene expression levels can be achieved proximal to the insertion site. By installing a unique 3-bp DNA barcode on each cassette, the different cassettes can be pulled in a single transposon library, probed for phenotype, and then de-multiplexed using the DNA bar code tag in a massively parallel fashion using next generation sequencing. In collaboration with Suzanne Walker’s laboratory (Department of Microbiology and Immunobiology, Harvard Medical School), we have identified a number of uncharacterized cell envelop related genes involved in resistance to clinically relevant cell envelope targeting antibiotics, and are currently determining their function. Using this approach, we recently identified the genetic determinants responsible for glycosylation of lipoteichoic acid (LTA), a key membrane bound lipid. LTA is a poly glycerol-phosphate chain attached to a diglucosyl-diacyl glycerol anchor that is specifically modified with N-acetyl glucosamine residues under cell envelope stress inducing conditions. We are interested in understanding how LTA glycosylation impacts cell envelope physiology.